Mick Costigan and Noah Flower contributed reporting to this article.
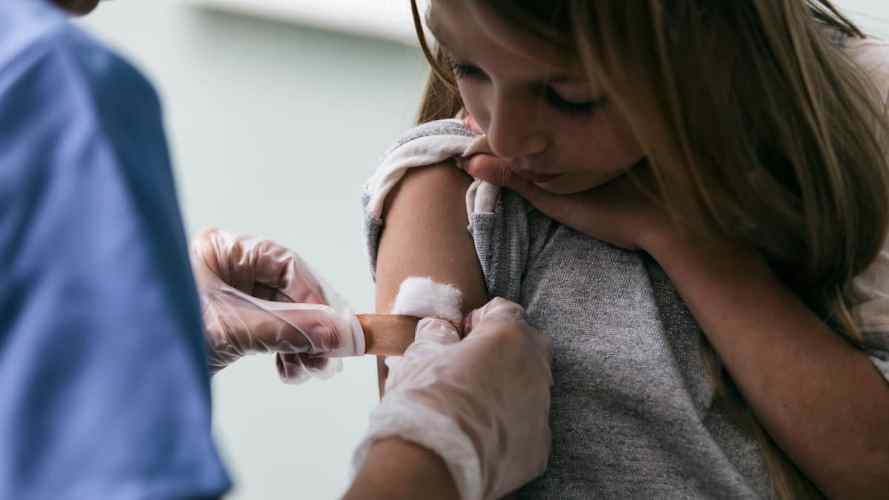
The public continues to digest a lot of new information about the global race for a COVID-19 vaccine and, as we enter the holiday season, we have some good news. Pfizer and Moderna recently reported their phase 3 vaccine trials were found to be at least 95% effective in preventing the disease. The companies plan to apply to the Food and Drug Administration (FDA) for emergency use authorization in the coming weeks. The federal government anticipates they can produce enough vaccine doses for 20 million people by the end of 2020, at which point distribution can theoretically begin.
The success of these trials is profoundly hopeful news. Only weeks ago, we were sitting with uncertainty about our chances of having a vaccine and whether the efficacy would be high enough to be helpful. Many business leaders are breathing a sigh of relief as a possible end to the crisis comes into view. They’re also looking for guidance as to how to plan ahead when things still feel uncertain in many ways. I’m hopeful that the scenarios we lay out here will help to that end.
An unprecedented vaccine in many ways
Producing a vaccine in such a short amount of time is historic, shattering the previous record of four years, set by the mumps vaccine. The reported efficacy levels are outstanding, far outstripping the 60-70% level typically achieved by flu vaccines. What’s more, the game-changing mRNA design of both vaccines is entirely new. It’s safer and more flexible than past vaccines and it opens up the additional exciting possibility of using similar designs to fight cancer.
It’s worth noting Moderna’s vaccine appears to have an edge over Pfizer’s in two ways. Long-term storage for the Moderna vaccine requires only -4 F, versus Pfizer’s requirement of -94 F. And for temporary storage, Moderna’s can be stored in a fridge for 30 days, versus five for Pfizer’s. Those differences will make a material difference to the cost and complexity of distribution, especially in the developing world. With other vaccine trial results yet to come, it’s possible other companies may solve these challenges.
Outstanding questions around the vaccine
Of course, several questions remain. The New York Times notes initial efficacy claims are not conclusive, since the findings have yet to be peer-reviewed — a process in which outside experts evaluate the detailed trial data.
Further, the results are based on symptomatic patients only. Whether the vaccine prevents asymptomatic infections, estimated to be up to two times the amount of symptomatic ones, is an open question. For both reasons, we could see the real efficacy figures reduced in the coming months.
Next comes the questions of making the vaccine available. The greatest current challenge is the Trump administration’s refusal to move forward with a formal transition to the incoming Biden administration. Because the rollout of the vaccine to 300 million Americans is such a monumental undertaking, Dr. Anthony Fauci said a smooth and efficient presidential handoff is key to making distribution fast and coordinated.
Finally, vaccines are not a magic wand providing instant relief from current transmission and illness. “Stopping a pandemic requires using all the tools we have available,” notes the Centers for Disease Control (CDC). But there may be an inclination for some people to ease up on precautions with a vaccine in view. Until we have contained the spread, it will be important for the public to maintain discipline with the non-pharmaceutical measures of masks, social distancing, and ventilation. See Salesforce advisor Tomas Pueyo’s latest article for a comprehensive summary of all the steps needed to reduce transmission.

What the vaccine news means for businesses
For those of us in charge of directing organizations, the range of uncertainty has decidedly narrowed. When my team and I developed our most recent scenarios to support critical decisions for organizations, I mentioned that in my nearly 50 years of scenario planning I’ve never seen uncertainty like that arising from the COVID-19 pandemic.
While things have moved in a positive direction, let us not jump to conclusions: it remains important to take an objective look at what uncertainty still remains before we make firm commitments.
Now, let’s take a deeper look at the three central uncertainties that define the track to a vaccine and the three potential outcomes that illustrate the new range of live possibilities. Any plans we make today should take this range into account.
Three pillars of uncertainty related to the vaccine
1. Timing of availability of an effective vaccine
Most conversations about a vaccine center on when one will arrive. In addition to the vaccines from Pfizer and Moderna, there are at least a half dozen other vaccines in development that have entered final (phase 3) trials or received partial approval.
Drug companies are manufacturing millions of doses in advance, so they can release them upon approval. The FDA guidance calls for COVID-19 vaccines to “prevent disease or decrease its severity in at least 50% of people who are vaccinated.”
This question of timing is now close to being resolved, as it is highly likely Pfizer and Moderna will receive FDA authorization. What’s more, the likelihood of other vaccines being approved has gone up, because both Pfizer and Moderna vaccines focus on the coronavirus’ “spike protein.” All of the other candidates supported by Operation Warp Speed also focus on that protein, so the early success of Pfizer and Moderna bodes well for the whole cohort, since they all take a similar approach.
How long will the vaccine last?
Relatively less attention has been paid to the question of a vaccine’s durability. With a novel pathogen, epidemiologists can’t predict how long immunity will last in individuals. A vaccine that lasts just a few months is barely useful, while one that lasts a year is much more so.
Two-year immunity is on the high end of expectations, since coronaviruses are “notoriously famous for not granting durable immunity,” in the words of Michael T. Osterholm, the widely-respected head of the Center for Disease Research and Policy (CIDRAP) at the University of Minnesota.
Fortunately, the prospects on this question just took a positive turn. A recent study found that antibodies to SARS-CoV-2 remain strong eight months after infection and are weakening so slowly they could remain for years.
Also important to containing the pandemic is whether a vaccine prevents infection or only illness. “We have to be very, very careful in making assumptions that … [a vaccine] will protect us from not only illness, but from infection,” said Osterholm. “We may not get a vaccine like that. We may ultimately get a vaccine one day that reduces the severity of illness.” It is not yet clear how the current vaccines will behave.

A road to a global vaccine
“A global program will be needed in over 200 countries,” said Dr. Larry Brilliant, a CNN medical analyst. “In order to achieve this, [the vaccine would need to enable] at least four years’ immunity via one single dose, rather than booster shots.”
Brilliant is a renowned epidemiologist who was part of the team that eradicated smallpox and also founder of Pandefense. He adds one final essential element: the vaccine will need to maintain its integrity without refrigeration in order for the World Health Organization (WHO) to have the best chance at controlling the pandemic. While the recent results are encouraging, they do not yet clear that bar.
Current expectations versus the historical norm
The widespread hope is now that the FDA approves emergency use authorization for the vaccines from Pfizer and Moderna in December, and the incoming Biden administration rolls out a coordinated national distribution plan, with large-scale vaccination beginning midyear. This represents the optimistic end of expectations among many epidemiologists, including Brilliant.
“I expect that by the new year we’ll have three to five vaccines that meet the low bar of 50% efficacy established by the FDA,” he told us in September, a prediction that now appears we will easily surpass.
Even in this rosy outcome, there are still concerns. Brilliant noted rare but dangerous side effects will not be discovered with only 30 thousand-to-40 thousand subjects getting the vaccine. Those could emerge down the road. Because typical rates of vaccine injury are roughly one per million, phase 3 trials could miss a problem that crops up at a rate of one per hundred thousand.
If bad side effects emerge, it will likely contribute to more anti-vaccination sentiment.
However, we should also remember the historical trend: experts note we’ve never released a coronavirus vaccine for humans before and, in any event, the fastest we’ve ever developed an entirely new vaccine was the four years it took to create one for mumps. By that timeline, we wouldn’t have a vaccine until 2024.
It is also important to realize that only about 30% of phase 3 trials for novel pharmaceuticals are successful. While optimism among epidemiologists has been high for some time that we will beat that timeline — and has gotten higher thanks to the recent news — those two facts should temper our expectations.
2. The challenge of getting a vaccine into people’s bodies
The challenge of vaccine development is matched only by the incredible complexities of production and distribution. In the U.S., the National Governors Association are among those raising questions, such as: how will it be quickly and efficiently delivered and dispensed? Who gets it first?
Complicating matters, the majority of vaccines under development will require two doses given 21 or 28 days apart, and two of the leading candidate vaccines have different handling requirements and must be stored at different temperatures; in Pfizer’s case extremely cold, at minus 94 degrees Fahrenheit. These types of cold chain requirements will likely slow rollout in the low- and middle-income countries as well.
To create herd immunity in a population, experts estimate that 60%-to-80% of the population will need to receive an effective vaccine. However they fear the decision to get a vaccine — similar to personal decisions around wearing masks and social distancing — will be influenced more by politics than science.
There’s already a general hesitancy about vaccines among “anti-vaxxers,” who have been heavily promoting their position. And many beyond that movement are sympathetic with the concern: two-thirds of Americans polled by USA Today/Suffolk Poll, say they won’t get a vaccine when it’s first available. Pfizer CEO Alberta Bourla warns that people who don’t take the vaccine will be the “weak link” that allows the virus to continue to spread.
An additional complication is that the emergence of a viable vaccine won’t mean public health measures like face masks, physical distancing, and routine hand washing can stop. Dr. Anthony Fauci told CNBC that even with a vaccine, many gaps in protection will remain. Even with a 90%-to-95% effective shot, “5%-to-10% of people immunized may get the virus,” he said. And, to counter this, the additional protective measures should last through 2021.
Depending on the gravity of these challenges, herd immunity may be a distant goal. The question is: how many people will overcome their hesitancy in pursuit of a normal life and how many will continue to fear the vaccine thanks to legitimate and manufactured evidence of risk?

3. Vaccine producers’ approach to distribution
Russia is calling its vaccine initiative Sputnik V. America named its effort Operation Warp Speed. As these monikers indicate, the race to develop a vaccine resembles the space race on steroids. Drug companies around the world are working on incredibly accelerated timelines, often subsidized by governments. The U.S. government, for example, has steadily contributed many billions since March to several companies developing vaccines and treatments.
Meanwhile other nations are already claiming vaccine advancements. On November 13, a South Korean biotech company agreed to produce more than 150 million doses of Russia’s Sputnik V vaccine, which it claims is 92% effective despite some controversy over the small size of the trial.
In September, the Wall Street Journal reported that a Chinese pharmaceutical company injected hundreds of thousands of people with experimental vaccines, as they and Russia “appear eager to start using their homegrown vaccines.” In addition to restarting their economies, these countries could be seeking to claim a public relations victory. Priority access to vaccines may be used as a tool, by them and by others, to attract allies on contested global issues like trade, energy, defense, and 5G technology.
Ramped up distribution
To address the global demand, vaccine manufacturers plan to ramp up their distribution networks, even designing new ones where necessary. Pfizer, for example, designed its own reusable cold storage boxes that can hold up to 5,000 vaccine doses for 10 days at ultracold temperatures. The company has reserved space on up to 20 FedEx and United Parcel Service planes each day. According to the Wall Street Journal, total time from distribution center to point of use will be about three days.
“Ensuring over a billion people globally have access to our potential vaccine is as critical as developing the vaccine itself,” Bourla told the paper in October.

Three possible outcomes
These uncertainties can be combined to create a range of plausible scenarios that highlight the many possibilities to consider. When one considers the flurry of activity around vaccine development, it may seem prospects are rosy, but we still have a believable path to many less-than-ideal outcomes.
If and when a vaccine arrives, some of the details will doubtless be different than any of the particular scenarios below. We offer them in an attempt to illustrate the range of variation that leaders should plan for, and most importantly, to drive home the importance of keeping tabs on what possibilities are currently on the table. If an outcome could plausibly occur, and it would have a meaningful impact, it is a leader’s responsibility to have a contingency plan at the ready. That preparation is what allows us and those whom we serve to move forward with confidence.
We’ve chosen to highlight three of these possible futures, including the most hopeful, which thankfully is now also appearing to be the most likely.
Three short-term COVID-19 scenarios to inform your business decisions
From recovery to depression, Chief Futurist Peter Schwartz explores the potential outcomes.



Scenario 1: “Zero Hurdles”
In this most-optimistic scenario, both the Pfizer and Moderna vaccines are approved in December, and the two companies release comprehensive study data that fully substantiates their earlier claims of 95% efficacy. The U.S. administration, under new leadership, reverses its current position and decides to work through COVAX, the multilateral arrangement designed to ensure orderly and equitable global distribution. Meanwhile, three additional vaccines successfully clear their phase 3 trials, bringing the total range of western options to five.
COVAX brokers deals for the vaccine’s creator to license production around the world, allowing a rapid ramp up of global manufacturing early in 2021, anchored by the large capacity of India’s Pune Serum Institute. Distribution runs smoothly and even low-income countries have easy and timely access, achieving at least selective vaccination where mass vaccination is not achievable. Innovations like smart labels on vaccine vials reduce spoilage due to heat exposure in doses that must be stored at ultracold temperatures.
Thanks to unified leadership, messaging, and investment from a wide range of international organizations, public trust in the vaccine is high. Fortunately, all five vaccines turn out to offer four-year immunity. We are able to achieve herd immunity and significantly reduce transmission by early 2022.

Scenario 2: “Sprint and Stumble”
China and Russia are first to emerge with vaccines, after testing them on their militaries, and begin offering access in December to low- and middle-income countries to build their global support base. Meanwhile, Moderna and Pfizer’s positive results yield emergency FDA authorization. Production scales up quickly and the U.S. provides free access to all in the early summer of 2021, creating a climate of heady optimism that the pandemic is all but finished.
Then the Russian vaccine begins to show issues with efficacy, as large numbers of Filipinos who received it begin to get reinfected, causing other countries to back away from their plans to rely on it. Next to emerge are problems with the Chinese vaccine, which appears to cause fatal complications for pregnant mothers.
Only weeks later, flaws in the vaccines come to light as the news breaks that the Moderna vaccine is causing heart attacks in people over forty, leading demand to shift to the Pfizer vaccine until it is revealed that it is causing mysterious deaths in the elderly.
These developments significantly expand the global anti-vaxx movement during 2021, which amasses large numbers of celebrity evangelists and deep-pocketed supporters, including the growing ranks of America’s QAnon. Its growth is further accelerated by the misinformation sown by Russian bots. While at least one of the existing vaccines appears safe for most of the population, rumors fly that big pharma and government are conspiring to hide the worst risks. Across the globe, a stalwart fact-based minority do accept the vaccine by 2022, but even by 2025 the numbers don’t clear the threshold of herd immunity.
Scenario 3: “Long March”
Repeated disappointments exhaust the public: first Pfizer’s vaccine, then Moderna’s, then other promising candidates turn out to be a mirage, ultimately failing to provide the needed combination of efficacy and durability. At long last, a usable vaccine is finally approved early in the summer of 2022. Fortunately, this is the vaccine that will serve the world: it appears to confer long-lasting immunity and can be administered as a simple spray, allowing anyone to be a vaccinator.
It gets rolled out around the world much like any other vaccine, with COVAX working with countries around the world to ramp up local production. Several countries nearly eradicate the virus within 18 months, and a larger group is successful within 2 years. The virus is never fully eradicated, as it continues to persist in the most unstable parts of the world, but for most of us life can finally move on.
Stress-test expectations
There is no way to know now which vaccine(s) will work which is why, as the Guardian noted, “wealthy countries are paying upfront for something that has not yet been proven to work [and are] willing to spend whatever it takes to get their economies running again. And yet, they could back the wrong horse. It’s a lottery on an unprecedented scale.” As we all play that lottery, we hope these stories give organizations a framework within which to stress-test their own expectations, improve their plans, and navigate today’s unprecedented uncertainty with confidence.
Work.com for Vaccines
Salesforce’s Work.com for Vaccines provides a rapid, safe, and flexible approach for managing, delivering, and administering vaccine programs.



Editor’s Note (Nov. 20, 2020): This post was originally published on September 18, 2020 and has been updated to reflect the most recent COVID-19 vaccine developments.
Editor’s Note (Sept. 18, 2020) : The hypothetical scenarios presented above are intended for business planning purposes only.